Rust can harm the appearance and surface value of an item, but it can also do major damage if it is not discovered and prevented from the beginning. Don’t let corrosion result in the failure of your product. Whether it leads to unhappy customers or a major structural issue in a building, it is important to know what corrosion is and the basics of preventing different types of corrosion.
How Corrosion Happens
Corrosion is primarily caused 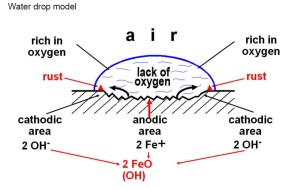 by moisture and the electrochemical reactions that happen between the fastener and it’s mating components. When a water drop rests on a plain iron surface and its surface is exposed to air, the surface is made rich in oxygen. The inside of the water droplet lacks oxygen resulting in electrochemical differences. This allows for an electric current to flow through the water acting as an electrolyte, causing iron ions to dissolve. While this is happening, hydroxide ions are also forming in the water and react with the iron ions causing precipitation of iron Hydroxide Fe which quickly forms iron oxide hydrate, or rust.
by moisture and the electrochemical reactions that happen between the fastener and it’s mating components. When a water drop rests on a plain iron surface and its surface is exposed to air, the surface is made rich in oxygen. The inside of the water droplet lacks oxygen resulting in electrochemical differences. This allows for an electric current to flow through the water acting as an electrolyte, causing iron ions to dissolve. While this is happening, hydroxide ions are also forming in the water and react with the iron ions causing precipitation of iron Hydroxide Fe which quickly forms iron oxide hydrate, or rust.
Types of Corrosion
To understand how to prevent corrosion, it is important to know the difference between the different types of corrosion. Knowing which type of corrosion you are dealing with will help to best determine how it can be prevented.
Uniform Corrosion: The most common type of corrosion, uniform corrosion is identified by its reddish color distributed evenly throughout the exposed part of the fastener.
Prevention: To prevent uniform corrosion it is important to allow for water run-off and good ventilation when designing. Also, prevent continuous condensation by keeping surfaces clean and protect fasteners from the beginning with platings or coatings.
Crevice Corrosion: Crevice corrosion occurs because small gaps and recesses tend to draw in moisture and do not have good ventilation. When crevice corrosion occurs, the risk for crevice corrosion multiplies with the number of joint faces.
Prevention: Crevice corrosion can be avoided by minimizing the use of washers and making joint interfaces as smooth as possible. Another strategy which helps is adding a gasket or sealer between the clamped materials.
Galvanic Corrosion: Galvanic corrosion is a result of two dissimilar metals in the presence of moisture.
Prevention: This type of corrosion can be prevented by using fastener materials or protective finishes that are either as noble or more noble than the joint. You can also use plastic washers where clamp load is not critical or avoid using stainless steel or copper parts with zinc plated fasteners.
Pitting Corrosion: Pitting corrosion occurs on a metal surface that is coated by a very noble finish like nickel or chromium. The exposed area becomes less noble than the much larger area around it that is less affected by environmental factors. This creates a current density allowing for galvanic corrosion in the pits.
Prevention: To prevent Pitting Corrosion always keep surfaces clean and smooth. Avoid solid or liquid residues, especially chlorides and use A4 or 316 stainless steel when there can be chlorides present. Also, work with your nickel plater to use subsequent treatments that can fill pores.
Intergranular Corrosion: Austenitic stainless steels can develop intergranular corrosions when they are heated to a high temperature for hot forming or welding.
Prevention: For intergranular corrosion prevention, use stainless steel with carbon content below 0.05% and quench parts in water immediately after heating. If using stainless steel containing over 0.05% carbon it can be stabilized by adding titanium, niobium or tantalum.
Stress Corrosion Cracking: Stress corrosion cracking may result when corrosion occurs on
fasteners subjected to tension. This type of failure often starts with pitting corrosion.
Prevention: Stress corrosion prevention can start with following the guidelines for pitting corrosion prevention. In addition to those guidelines, periodically inspect safety critical parts, hot dip galvanizing if necessary, to check for corrosion. Always ensure that these safety critical fasteners are accessible for inspection and replacement.
Hydrogen Embrittlement: While not a form of corrosion Hydrogen embrittlement in a fastener can be a result of corrosion in a joint. If high strength fasteners are stressed, small surface defects like scratches may turn into a small crack. If hydrogen is present in the steel atoms, they are attracted by the tensile stresses around the tip of the crack and form a “hydrogen atom cloud” there. The hydrogen weakens the microstructure of the metal and the crack may continue to grow until the part fails.
Prevention: To prevent Hydrogen embrittlement, avoid electroplating or the use of acid cleaning for high strength fasteners. If Hydrogen is introduced in large quantities to a high strength fastener the possibility of embrittlement is very prominent.
Above all, make fastener choice a priority during the initial stages of the design processes to avoid chance of corrosion from the start.
Have any corrosion stories?
Share them below!
To learn more about corrosion and preventing corrosion, contact us at ProvenProductivity@bossard.com.
What is Corrosion and How Can You Prevent It? by Bossard




